Background: Patients with myelodysplastic syndromes (MDS) undergo risk stratification with various prognostication tools, such as the International Prognostic Scoring System (IPSS) and Revised IPSS (IPSS-R). Recently, the Molecular IPSS (IPSS-M) was developed incorporating genomic data in addition to clinical and cytogenetic characteristics. Though IPSS-M has been shown to improve prognostication in all MDS patients at baseline, its utility in specifically patients undergoing hypomethylating agent (HMA) therapy remains unknown. Here, we assess the efficacy of IPSS-M in HMA-treated patients with MDS.
Methods: We retrospectively evaluated all untreated patients with MDS seen at a single tertiary cancer center from July 2017 to July 2021 and identified those who later received HMA therapy. Patient characteristics and bone marrow (BM) data, including morphology, cytogenetics, and mutations, were assessed at diagnosis. Genomic DNA was extracted from whole BM aspirate samples and subject to 81-gene target PCR-based sequencing using a next-generation sequencing platform. Survival data was updated in July 2023.
Results: Out of 799 untreated MDS patients, 455 patients (57%) eventually underwent treatment with HMA. At diagnosis, the median age was 69 (range: 22-90) with 289 (64%) male patients. Patients were generally high risk, with therapy-related MDS in 160 (35%), complex cytogenetics in 167 (37%), and TP53 mutations in 168 (37%). By IPSS-R, 259 patients (58%) had high- or very high-risk disease, and 340 patients (75%) had higher-risk disease (moderate high-, high-, and very high-risk) by IPSS-M. A total of 291 patients (64%) were treated with HMA monotherapy, and lower-risk MDS patients received more cycles of HMA (p < 0.001).
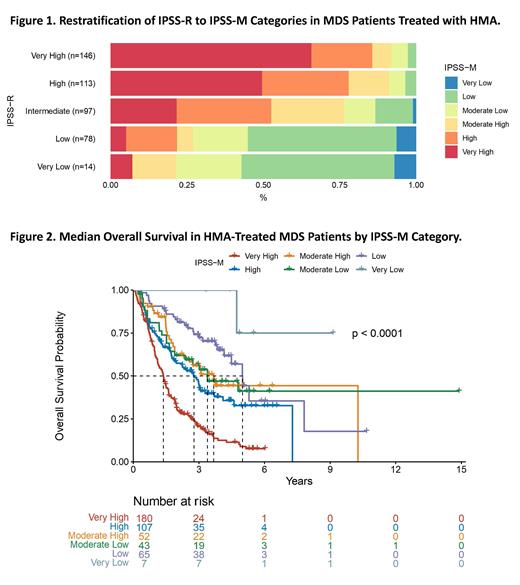
By IPSS-R, the median overall survival was 60.0 months (95% CI: 46.0, not estimable (NE)) in very low-, 87.4 months (95% CI: 59.9, NE) in low-, 36.2 months (95% CI: 30.0, 58.1) in intermediate-, 23.7 months (95% CI: 19.6, 34.8), in high-, and 12.8 months (95% CI: 11.0, 16.7) in very high-risk patients (p < 0.0001) with a concordance index of 0.669. Figure 1 depicts the reclassification of patients between IPSS-R and IPSS-M. Risk stratification by IPSS-M (Figure 2) provided similar results: not reached (95% CI: 56.8, NE) in very low-, 60.0 months (95% CI: 49.8, NE) in low-, 40.8 months (95% CI: 22.8, NE) in moderate low-, 44.2 months (95% CI: 27.8, NE) in moderate high-, 33.4 months (95% CI: 22.8, 44.5) in high-, and 16.6 months (95% CI: 13.8, 19.4) in very high-risk patients (p < 0.0001). The concordance index of IPSS-M in all HMA-treated MDS patients was 0.648. When analyzing patients with MDS who were more likely to undergo HMA as per standard-of-care treatment (those with IPSS-M moderate low-, moderate high-, high-, and very high-risk disease), the IPSS-M remained statistically significant (p < 0.0001) though less discerning in the patients with moderate-risk MDS, as seen by the concordance index of 0.620.
Conclusions: In this group of MDS patients treated with HMA, the IPSS-M did not provide additional prognostic power over the IPSS-R though median overall survival remained inversely proportional to IPSS-M risk category. Concordance indices for survival remained below 0.7, likely due to the overlapping curves in moderate-risk patients by IPSS-M. Further development and validation of prognostic scoring systems in patients treated with HMA are warranted.
Disclosures
Chien:AbbVie: Consultancy; Rigel Pharmaceuticals: Consultancy. Montalban-Bravo:Rigel: Research Funding; Takeda: Research Funding. Short:Takeda: Consultancy, Research Funding; AstraZeneca: Consultancy; Astellas: Research Funding; Stemline therapeutics: Research Funding; Novartis: Consultancy; Pfizer: Consultancy; Amgen: Honoraria. Jabbour:Takeda: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Adaptive Biotech: Consultancy, Honoraria, Research Funding. Kadia:Pulmotect, Inc.: Consultancy, Research Funding; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Delta-Fly Pharma, Inc.: Research Funding; Sanofi-Aventis: Consultancy; Glycomimetics: Research Funding; Regeneron Pharmaceuticals: Research Funding; Agios: Consultancy; SELLAS Life Sciences Group: Research Funding; Genzyme: Honoraria; Servier: Consultancy; Pinotb-Bio: Consultancy; Janssen Research and Development: Research Funding; Astellas Pharma Global Development: Research Funding; AstraZeneca: Research Funding; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Novartis: Consultancy; Genentech: Consultancy, Research Funding; GenFleet Therapeutics: Research Funding; Amgen, Inc.: Research Funding; Liberum: Consultancy; Iterion: Research Funding; Pfizer: Consultancy, Research Funding; Ascentage Pharma Group: Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Cyclacel: Research Funding; Celgene: Research Funding; Cellenkos Inc.: Research Funding; Cure: Speakers Bureau; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria. Ravandi:Syros: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Biomea fusion: Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Research Funding; Amgen: Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding. Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend:: Membership on an entity's Board of Directors or advisory committees. Kantarjian:Ipsen: Honoraria; Jazz Pharmaceuticals (Inst): Honoraria, Research Funding; Immunogen (Inst): Honoraria, Research Funding; Abbvie (Inst): Research Funding; Astellas Pharma: Honoraria; Shenzhen Target Rx: Honoraria; Pfizer: Honoraria; Ascentage Pharma Group: Honoraria; Amgen: Honoraria; Amgen (Inst): Research Funding; Abbvie: Consultancy, Honoraria; Novartis: Honoraria; Novartis (Inst): Research Funding; Taiho Pharmaceutical: Honoraria; KAHR Medical: Honoraria; Ascentage Pharma (Inst): Research Funding; Bristol-Myers Squibb (Inst): Research Funding; AstraZeneca/MedImmune: Honoraria; Daiichih-Sankyo (Inst): Honoraria, Research Funding; Precision Biosciences: Honoraria. Garcia-Manero:Bristol Myers Squibb: Other: Medical writing support, Research Funding; Genentech: Research Funding; AbbVie: Research Funding.